***Beta version***
For now, this version of the application contemplates the technical content aimed at health professionals. Soon, the Ministry of Health will make available, for each medicine, the content with terms adapted to better understand the citizens.
At that moment, the Ministry of Health is working to resolve the inconsistencies already identified.
Access the form at the following link to report errors, inconsistencies, or suggestions for improvements, as well as adjustments to the content of drug information.
Link: https://goo.gl/eZ5nHA
---
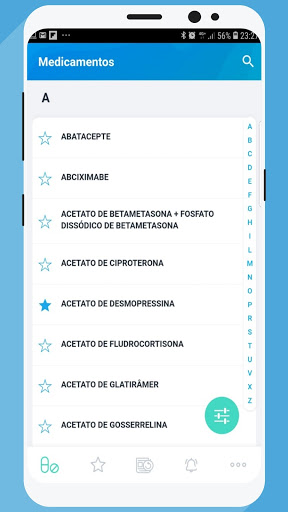
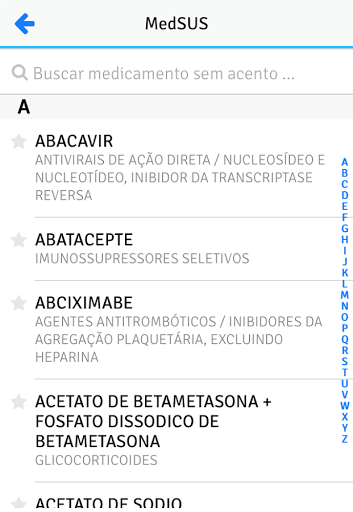
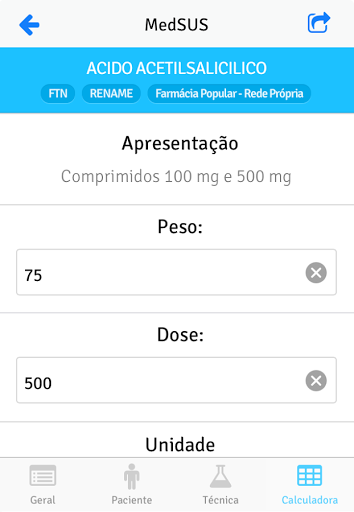
MedSUS is an application made available by the Ministry of Health and which gathers information about the medicines contained in the National List of Essential Medicines (Rename) of the Unified Health System (SUS).
The goal of MedSUS is to facilitate access to information on medicines by health professionals and citizens, to promote the rational use of medicines.
- FTN and Rename Digital
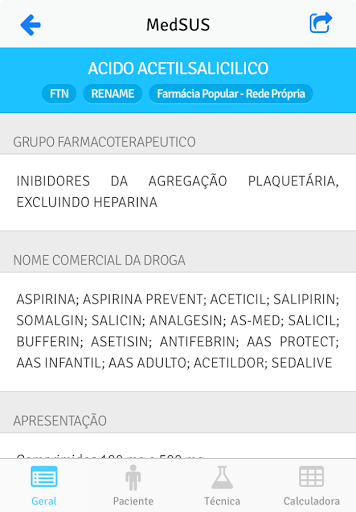
The information comes from the monographs of the National Therapeutic Form (FTN), reference work that accompanies the Rename and that subsidizes the prescription, dispensation and rational use of medicines.
- Quality scientific information
The monographs have a number of evidence-based, high-credit sources of information, such as WHO drug forms, the Ministry of Health clinical guidelines, and international databases.
- Complete information
The application contains information about the drug as active principle, presentation, indication of use and administration schemes, adverse reactions, drug interactions, among many others.
- Constant Updates
Drug monographs will be updated each time the application is opened. Also included will be the inclusion and exclusion of medications by SUS.
- Share
Share the monographs of the medicines of your interest and make it easier to use the information on a daily basis.
- Favorite
Mark the medicines that you most access and make it easy to use the application.
Filter
Optimize your searches using the Rename Attachment, Special Control, Major Anatomical Group, and generic drugs.
 Samsung Galaxy Grand Prime
Samsung Galaxy Grand Prime